Gene Synthesis & Cloning/Mutagenesis FAQs
The Most Commonly Asked Questions
| Service | Service Offering | Estimated Turnaround Time | Price |
| FragmentGENE | Synthesis of double-stranded DNA fragments | 2-4 business days | $ |
| PriorityGENE | Standard gene synthesis with cloning into a plasmid | 8-10 business days | $$ |
| TurboGENE 5 | Expedited gene synthesis with cloning into a plasmid and our fastest available turnaround | 5 business days | $$$ |
| TurboGENE 7 | Expedited gene synthesis with cloning into a plasmid | 7 business days | $$$ |
| Antibody DNA Synthesis | Synthesis of heavy and light chain sequences with cloning into a plasmid | 6-8 business days | $$ |
| AAV Plasmid Synthesis | Synthesis of AAV sequences and cloning into a plasmid with AAV-ITR sequence verification using proprietary technologies | 11-15 business days | $$$ |
| CRISPR Construct Synthesis | Synthesis of gRNA and cloning into a custom plasmid | 8-10 business days | $ |
| ssDNA Synthesis | Synthesis of single-stranded DNA fragments | 10-15 business days | $$ |
PriorityGENE, TurboGENE, ValueGENE, antibody DNA synthesis, and AAV plasmid synthesis services require you to request a quote before ordering. FragmentGENE, CRISPR construct synthesis, and ssDNA synthesis services are available for direct order, so you do not need to request a quote for these services.
Log in to your online GENEWIZ account and select “Gene Synthesis”, then click on the name of the specific service you are interested in. You will be directed to a quote request form for that selected service. Once you complete the form, select the “Review Inquiry” button at the bottom of the screen. After you review the entered details, select the “Submit Quote Request” button to request your quote. Upon submission of the inquiry, you can expect to receive a quotation within one business day. You can view a PDF copy of the order information including price and download using the “Print Price” button.
The assembled full-length gene is cloned into the pUC-GW-Kan/Amp vector via the EcoRV site. The final construct is verified with both Sanger DNA sequencing (on at least one strand) and restriction digestion. The sequences of pUC-GW-Kan/Amp can be found here.
Please submit all required starting material to GENEWIZ. For UK projects (40-) samples should be submitted to our UK subsidiary, and for continental Europe projects (50-/90-) samples should be submitted to our German subsidiary (addresses below) as soon as possible after initiating your order (if required). A detailed list of the required starting material is included in section III “How to start your order” of your gene synthesis quotation.
Kindly submit a 5µg aliquot (minimum concentration of 20ng/µL) of the undigested, purified, circular plasmid. Please ensure that the name of the plasmid on the tube matches the name submitted for the order. Include the first page of the order receipt or quote with the submitted plasmid sample to ensure they are accurately identified
For UK: DNASeqUK@genewiz.com | (+44 (0) 1279 873837)
For EU: Logistics.Europe@genewiz.com | (+49-341 520 122 – 41)
All vectors submitted to our facility will be held in storage for two years and available within this time for future project use.
Direct Shipping (FedEx, UPS & DHL):
If a GENEWIZ dropbox is not available, or if your starting material requires dry ice, please use an overnight delivery service such as FedEx, UPS or DHL.
UK Shipping Address:
Azenta
Attn: Project Management
Hope End, Takeley
Essex CM22 6TA
United Kingdom
Tel: +44 01279 873837
Germany Shipping Address:
Azenta
Attn: Project Management
Bahnhofstrasse 86
04158 Leipzig,
Germany
Phone: +49-341 520 122-41
GENEWIZ dropbox (If Available):
Starting material that is stable at room temperature may be submitted via a GENEWIZ dropbox (when available). Contact dnasequk@azenta.com (UK) or Logistics.Europe@azenta.com (continental Europe) to request a dropbox pick-up for your location, or if you have any related questions. Please click here for other dropbox options.
We store any starting material provided and final constructs generated at our facility for up to two years to be used for future orders. For more information, please find our Sample Storage Policy here.
The timeline will depend on the size of the gene to be synthesized, the complexity of the vector for cloning, and the requested DNA preparation scale. For reference, the turnaround time for a gene less than 1.5 kb is 8-10 business days with our PriorityGENE service, 7 business days with TurboGENE 7 priority, or 5 business days with TurboGENE 5 priority. A more specific turnaround time for each order is included within the quotation, as well as the “Order Review” page within your online account.
GENEWIZ sample tubes are delivered containing 2-5 µg lyophilized plasmid DNA for gene synthesis orders, or 500 ng – 1 µg purified linear dsDNA for FragmentGENE orders. For resuspension, we recommend the following:
a. Spin the tube briefly to ensure that the contents are on the bottom of the tube
b. Resuspend the sample in a volume appropriate for your needs:
i. For example, 20-50 µL for ~0.25 to ~0.1 µg/µL of sterile high purity water
c. The resuspension solution is dependent on downstream applications – TE may interfere with various enzyme manipulations
d. Vortex briefly, let the DNA tube sit for 2-10 minutes on ice, then vortex again
ORDERING
Alternatively, please feel free to download our Ordering Template and fill out the necessary information within this form. To access this form within a gene synthesis inquiry, select the “Download/Upload” button located at the top right corner of the screen. After this is done, select the same “Download/Upload” button to view the option to upload the completed form.
Select the “Review Quote” button next to the related project tracking number (30-xxxxxxxxx). After this is done, an “Add to Cart” button will appear at the bottom of the screen. Once this is selected, you will be redirected to the ordering page where you will be able to review our Terms and Conditions and enter the payment information for this order. After this is done, select the “Confirm” button at the bottom of the screen to place your order.
Our Project Management Team is happy to help with any questions. Please feel free to contact us from 9:00 AM – 6:00 PM CET via phone (+49 (0) 341 520 122-41), email (gs@azenta.com), or live chat.
Our antibody DNA synthesis service provides synthesis and cloning of your antibody heavy/light chain sequences up to 1.5 kb into any custom vector in as few as 6 business days, the fastest turnaround time on the market. Our PriorityGENE service provides synthesis, assembly, and cloning of sequences with unlimited length on a timely basis.
With our AAV plasmid synthesis service, we can synthesize and clone transgene expression cassettes into custom AAV vectors with high efficiencies. All final products will come bundled with mini-scale or large-scale DNA preparation using our new AAV plasmid preparation protocol and AAV-ITR sequence verified AAV plasmids. This service also offers ITR correction if mutations are found after sequence-verification of these regions.
SERVICE DETAILS
GENEWIZ will synthesize cloned gene sequences ranging from 1 bp to 50+ kb, using short DNA oligos as building blocks.
GENEWIZ has a proven track record of synthesizing difficult templates, such as GC-rich sequences and sequences with repeats, using our proprietary technologies. With our bioinformatics platform, GENEWIZ optimizes your gene for synthesis.
In some very rare instances, extreme sequence content may be refractory to available sequence verification techniques. GENEWIZ carefully reviews every sequence to estimate difficulty and anticipate project challenges and customizes every project quotation to include an estimated turnaround time and pricing information based on this analysis.
GENEWIZ provides a custom quote for every gene synthesis project. For standard priority, typical gene sequences (non-repetitive, average GC-content) that do not require custom subcloning, no additional fees will apply. For genes with expedited handling, difficult sequences, large inserts, or for projects with custom subcloning requirements, any relevant fees will be indicated in your custom gene synthesis quote.
At GENEWIZ, the protection of our customers’ intellectual property is a top priority. Proprietary information is always under client ownership, and GENEWIZ places the utmost importance on confidentiality and privacy. Comprehensive protective measures enable GENEWIZ to earn and maintain the trust and confidence of all customers, collaborators, and partners. For more information about the GENEWIZ Confidentiality Policy, please click here.
As the variants are being created from the parent sequence, we can offer a discounted price for variant synthesis. Please keep in mind, variant synthesis does add an additional 3-5 business days to the quoted turnaround time.
RECOMBINANT ANTIBODY PRODUCTION
Yes, we offer a pETE vector which is optimized for our recombinant expression platform. You can provide your own preferred expression vector as well.
Yes, we perform antibody affinity characterization by surface plasmon resonance (SPR) and bio-layer interferometry (BLI).
Expression analysis is performed by BLI (Octet) to estimate the amount of protein in the supernatant. Non-producing plasmids will be reported to the customer and excluded from purification, so you do not pay purification fees on non-producing constructs.
Standard deliverables include: 2-5ug synthesized DNA construct (if performed by GENEWIZ), antibodies provided as purified protein or supernatant, Certificate of Analysis (CoA)
LENTIVIRUS
Lentivirus, meaning “slow virus”, is a genus of retrovirus. Retroviruses express reverse transcriptase to convert their RNA genome into double-stranded DNA (dsDNA) and integrase to incorporate this viral DNA into the host’s genome. Due to this permanent DNA integration, the host cell produces more retroviruses as it divides, resulting in long-term expression of the disease.
Lentivirus has long incubation periods between host infection and the onset of disease symptoms, such as human immunodeficiency virus (HIV) in the case of human acquired immune deficiency syndrome (AIDS). In biotechnological applications, recombinant lentiviral vectors are modified for safe handling and used as a gene delivery vector for mammalian cells.
Unlike other retroviruses that only infect dividing cells, lentiviruses infect both dividing and non-dividing (postmitotic) cells. This broad tropism, coupled with the stable integration into a host cell genome, allows for diverse in vitro and in vivo applications, including clinical cell therapy and CAR-T cell engineering. Additional advantages of lentivirus include the ability to express large transgenes (up to 9 kb) and low immunogenicity.
Our team of experts uses HEK293T cells for lentiviral vector production. Following successful transfection with four packaging plasmids, the cell machinery facilitates the assembly and replication of the lentiviral particles during an approximately three-day incubation. Lentivirus particles are then harvested, filtered, purified, concentrated, and aliquoted to small volumes to facilitate your downstream transduction applications.
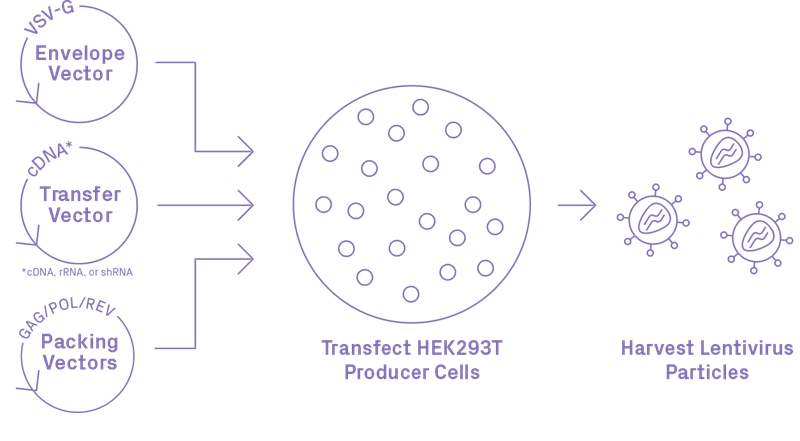
Recombinant lentiviral vectors are generally considered safe as they are engineered to be replication incompetent, with the third-generation considered the safest. It features a chimeric 5’ LTR, self-inactivating truncated 3’ LTR, elimination of Tat, and a four-plasmid transfection system that has a low likelihood of generating a replication-competent virus. According to the guidelines of the National Institutes of Health (NIH), lentiviral particles should be handled as Risk Group 2 (RG2). Our team uses the third-generation packaging system in biosafety level 2 (BSL2) facilities for all default lentiviral handling.
Yes! Upon review, our expert team can accommodate custom requests. Our default option is an envelope glycoprotein of vesicular stomatitis virus (VSV-G protein).
Yes! Our team of experts have extensive experience packaging various library types, including combinatorial and trimer-controlled libraries. The diversity of the final packaged lentivirus will depend on the plasmid material. If you do not already have a library sample, we can generate and validate this for you. Please see here for more information.
Yes! We offer various transfer plasmid options for your cloning needs in the context of lentivirus production. If we don’t have a vector that suits your requirements, we can synthesize any necessary components to create a transfer plasmid that aligns with your design.
We offer two QC options for the validation of your lentiviral particles:
1. p24 ELISA (physical titer): This method, offered as our standard QC, utilizes a sandwich ELISA to detect the presence of p24 protein, a major capsid protein encoded by the Gag gene. The p24 protein level is measured spectrophotometrically against a standard curve. Next, the quantified p24 value is correlated to the virus particle number. To facilitate downstream applications, we convert the lentiviral particles (LP) to TU/mL by dividing by 100 (there are ~100-1000 LPs for every active virus). This method is considered as a measure of physical titer.
2. WPRE qPCR (functional titer): An aliquot of the packaged lentivirus vector is used to transduce HEK293T cells at various dilution levels. After a two-day incubation period, the genomic DNA of the transduced cells is harvested. Following that, qPCR amplification of the WPRE element is then performed alongside a calibrated standard curve. The WPRE element is absent in wild-type HEK293T cells but exists within the lentiviral cassette of a transfer plasmid. As the WPRE elements can only be detected in HEK293T cells if transduction is successful, this method is considered as a measure of functional titer.
Physical titering approximates the total amount of virus present in the sample by correlating it to the spectrophotometrically quantified p24 in an ELISA assay, regardless of whether it possesses the gene of interest or ability to infect cells.
Functional titer is a measure of virus infectivity, quantifying the copy number of the WPRE element, which is detectable only upon a successful viral DNA integration into the host cell genome. Generally, functional titer is 100-1000-fold lower than physical titer. We report physical titer after accounting for this ratio, and express p24 in TU/mL.
Pseudotyping is the process of incorporating envelope glycoproteins from other viruses to generate lentivirus particles with altered tropism. This is often used to enhance the infectivity and stability of lentivirus. For example, VSV-G is commonly used for pseudotyping due to its broad tropism.
CODON OPTIMIZATION
GENEWIZ codon optimization tool can optimize for multiple critical parameters to stabilize DNA fragments and improve gene expression efficiency. Parameters include, but are not limited to:
a. Codon usage bias
b. GC content
c. mRNA secondary structure
d. Custom desired patterns
e. Custom undesired patterns
f. Repeat sequences (direct repeat, inverted repeat, and dyad repeat)
g. Restriction enzyme recognition sites (deletion or insertion)
Please note, GENEWIZ cannot guarantee final protein expression.
Yes, we offer dual optimization for any two expression systems requested. Additional requirements are also considered for review by the project team. Please note that any dual-system optimization represents a compromise, and a sequence specifically optimized for any given system may outperform the compromise sequence. To add this to your order, please add the request to the “Order Comments” section of your gene synthesis inquiry or email the Project Management Team at gs@azenta.com.
CLONING AND MUTAGENESIS
We currently only provide our in-house pUC-GW-Kan/Amp vectors for cloning. If you’d prefer cloning into a different vector, an aliquot of your custom vector will need to be provided upon confirmation of your order.
For any information regarding on-going promotional discounts, please contact your sales representative.
| Site-Directed Mutagenesis | Insert PCR-Based Mutagenesis | TurboMUTANT | |
| Plasmid Size Guidelines | 10kb or less in size, Non-complex |
> 10 kb/unstable plasmid | < 8 kb/stable plasmid |
| Turnaround Time | Starts from 8-10 BD | Starts from 10-15 BD | Starts from 5 BD |
QUALITY AND FINAL DELIVERABLES
Standard deliverables for GENEWIZ gene synthesis projects include:
a. 2-5 µg of lyophilized plasmid containing your desired synthetic construct
b. Certificate of Analysis (COA), including restriction digest
c. Sequence trace data with alignment
d. Sequence of synthetic gene alone and in the designated vector
All reagents, material used, and equipment are kept under strict quality controls by the lab. The Project Management Team may decide to implement additional controls if an uncommon failure rate is observed.
Mutations can be found at multiple stages of a project. For example, a synthesized primer may contain an incorrect base. Mutation correction may delay delivery by 1-4 business days depending on the situation.
The GENEWIZ Project Management Team is happy to address your additional project requests through a new quotation request that references the current open project. Where possible, any discounts applied to the current project will be incorporated into the new quotation. Please note that you must confirm the new project within the ongoing project timeline in order to take advantage of these discounts.
Yes, unless otherwise specified in the quotation due to extreme complexity. Single-strand sequence verification across the entire insert occurs for every synthesis product. If the sequence-verified synthesis product is associated with a large-scale preparation, the product from the large-scale plasmid preparation is sequenced across junctions. Additional sequence verification on the second strand, or after large-scale plasmid preparation, is available if requested (additional charges apply).
Some extremely complex sequences are expected to not be verifiable using current Sanger sequencing techniques and are noted in the quote. In the very rare event where the Project Management Team is not able to deliver the intended material, there will be no charges for your project (unless otherwise specified in the quotation due to extreme complexity).