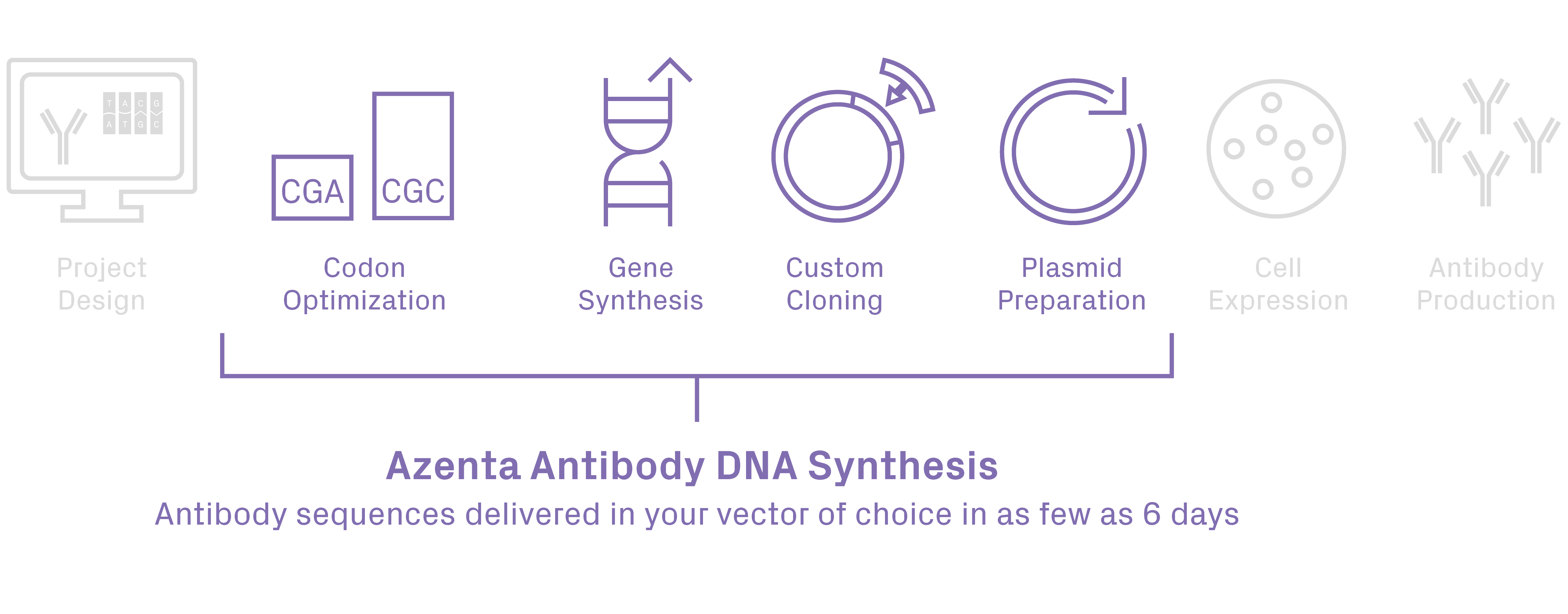
Antibody Synthesis
Antibody Synthesis service provides synthesis and cloning of your antibody heavy/light chain sequences into any custom vector in as few as 6 days. This is the fastest turnaround time on the market. Our high-quality results are paired with FREE recombinant antibody expression optimization and FREE cloning vector storage for up to 2 years.
GENEWIZ Multiomics and Synthesis Solutions from Azenta Life Sciences offers a customer-friendly antibody synthesis service with Ph.D.-level technical support to alleviate common synthesis obstacles and streamline your antibody discovery research.
What is the process of Antibody Synthesis?
Antibody synthesis is the production of antibodies – proteins created by the immune system of a host — in response to an antigen, a foreign body that enters the host. The production of an antibody involves several steps:
- Preparation of the immunogen
- Immunization
- Creation of a hybridoma
- Collection
- Screening
- Isotyping
- Purification of the antibody
- Antibody labeling for direct use
Antibody Synthesis Features & Benefits
FASTEST ANTIBODY DNA SYNTHESIS ON THE MARKET
| Synthetic Gene Length | Turnaround Time (TAT) |
| <500 bp | 6-8 Business Days |
| 501-750 bp | |
| 751-1500 bp | 8-10 Business Days |
| Custom cloning | No Additional TAT |
Synthesize all types of DNA Sequences for Antibody Research
Advancements in recombinant monoclonal antibody (rAb) technology have led to the development of a variety of engineered antibody molecules for research, diagnosis, and therapy. With over 10 years of expertise in gene synthesis and cloning, GENEWIZ has supported numerous antibody discovery and development projects, including researchers developing broadly anti-virus monoclonal antibodies [1], bispecific recombinant antibodies [2], anticancer Fc-engineered human monoclonal antibody [3, 4], and much more.

Figure 1. Synthetic DNA in antibody development
Recombinant antibodies can be developed using de novo synthesis of recombinant VDJ light chain and heavy chain sequences. Synthetic DNA can be also used in humanized or other recombinant antibodies such as single-chain variable fragment (scFv), single domain antibody (sdAb), fragment antigen binding mAb (Fab), trifunctional antibody, etc.
Synthesize all types of DNA Sequences for Antibody Research
Advancements in recombinant monoclonal antibody (rAb) technology have led to the development of a variety of engineered antibody molecules for research, diagnosis, and therapy. With over 10 years of expertise in gene synthesis and cloning, GENEWIZ has supported numerous antibody discovery and development projects, including researchers developing broadly anti-virus monoclonal antibodies [1], bispecific recombinant antibodies [2], anticancer Fc-engineered human monoclonal antibody [3, 4], and much more.

Figure 1. Synthetic DNA in antibody development
Recombinant antibodies can be developed using de novo synthesis of recombinant VDJ light chain and heavy chain sequences. Synthetic DNA can be also used in humanized or other recombinant antibodies such as single-chain variable fragment (scFv), single domain antibody (sdAb), fragment antigen binding mAb (Fab), trifunctional antibody, etc.
WHAT OUR CUSTOMERS ARE SAYING
“I have always had great customer service from GENEWIZ, received great products with a good price. It allows me to do other things in the lab instead of spending time cloning, etc.”
– Researcher from Cincinnati Children’s Hospital Medical Center
“Good quality, customer support, and expediated delivery to help me to meet the deadline.”
– Principal Investigator from a top pharmaceutical company

Application Note | Accelerating Recombinant Antibody Production
Recombinant antibody production is a cornerstone of modern biotechnology and research, but it faces the significant challenge of achieving high yields. In this application note, discover how to enhance expression efficiency while reducing production times with an optimized vector backbone workflow.
RELATED PUBLICATIONS
[1]Xiao H, Guo T, Yang M, et al. Light chain modulates heavy chain conformation to change protection profile of monoclonal antibodies against influenza A viruses. Cell Discov. 2019;5:21. Published 2019 Apr 16. doi:10.1038/s41421-019-0086-x
[2]Ahmed M, Lopez-Albaitero A, Pankov D, et al. TCR-mimic bispecific antibodies targeting LMP2A show potent activity against EBV malignancies. JCI Insight. 2018;3(4):e97805. Published 2018 Feb 22. doi:10.1172/jci.insight.97805
[3] Qi X, Li F, Wu Y, et al. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat Commun. 2019;10(1):2141. Published 2019 May 20. doi:10.1038/s41467-019-10088-1
[4]Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcγR Engagement. Cancer Cell. 2016;29(6):820–831. doi:10.1016/j.ccell.2016.05.001
