AAV Plasmid Preparation
Preserving the integrity of inverted terminal repeat (ITR) sequences in adeno-associated virus (AAV) plasmids is important for the production of recombinant AAV (rAAV) vectors. GENEWIZ proprietary AAV plasmid preparation service delivers superior quality for midi to giga-scale AAV vectors, while maintaining the integrity of ITR regions. Our new process includes pre- and post-preparation quality controlled (QC) steps that leverage GENEWIZ’s AAV-ITR Sanger sequencing capabilities for full sequence verification and ensures delivery of intact ITR clones for your research.

AAV-ANCED STRATEGIES
For Adeno-Associated Virus in Gene Therapy
✓ Reliable quality control and safety testing for AAV development
✓ Strategies to read through inverted terminal repeats in AAV
✓ High-fidelity methods for AAV plasmid prep and packaging
✓ Advanced sequencing for AAV quality and safety monitoring
Preserve ITR INTEGRITY in aav vectors
Truncated ITRs can reduce the yield of full viral capsid production and increase generation of undesirable non-rAAV encapsidated DNA. Unfortunately, AAV plasmid ITRs are frequently mutated during plasmid production in bacterial cells. GENEWIZ AAV plasmid preparation service incorporates a two-step QC process, including pre-prep and post-prep QC. The pre-prep QC step assesses your sample configuration for the presence of full-length intact or truncated ITRs, or a mixture of both. This allows selected propagation of intact ITR sub-populations only, while the post-prep QC step ensures integrity of ITR regions in the final plasmids delivered to you. For whole plasmid sequencing, utilize our primer walking service with AAV plasmid preparation.
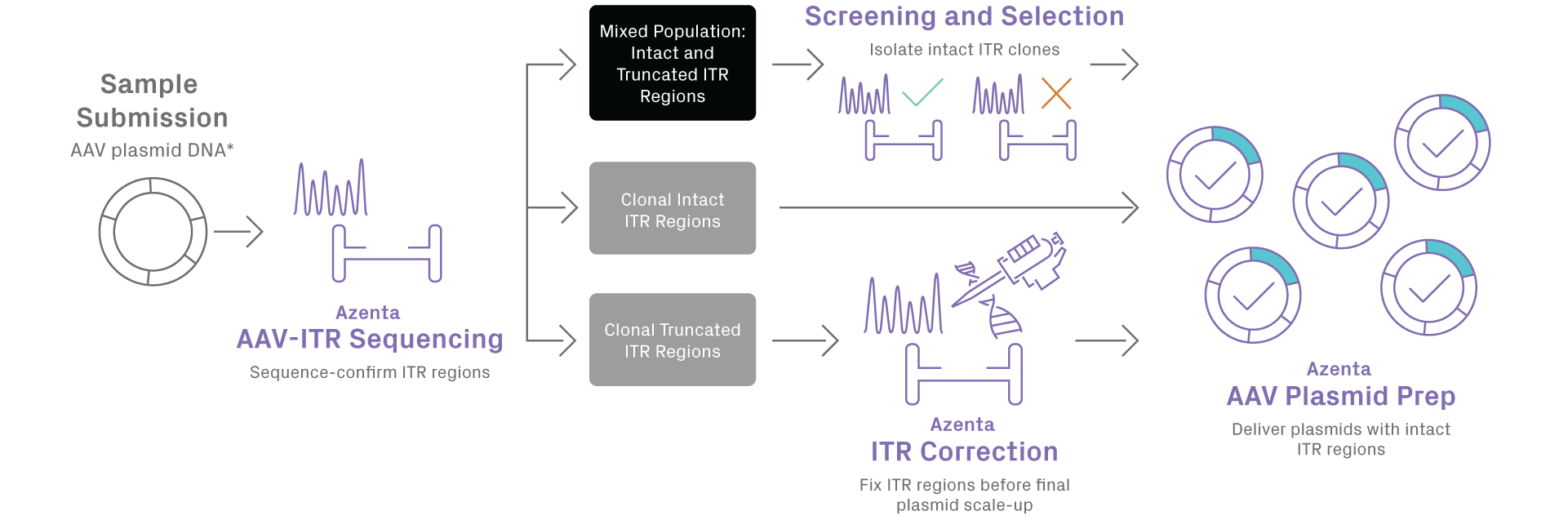
*Plasmid DNA is currently the only starting material accepted
Note: Screening and selection of intact ITR clones incurs additional cost
Features & Benefits
Turnaround time starting at 6 business days** with Ph.D.-level support throughout project.
High-quality midi- to giga-scale preps available.
High success rate compared to standard plasmid preparation kits and protocols.
Multiple analysis options available, including sterility testing, restriction digest, full plasmid sequencing, etc.
**6-day turnaround time for samples that have previously been ITR sequence verified at GENEWIZ.

Tech Note | Optimizing Plasmid Preparation to Increase Yield and Reduce Endotoxins
Plasmid DNA (pDNA) is vital for a range of biopharmaceutical and biotechnological applications but maximizing yield while minimizing endotoxins can be challenging. This tech note explores strategies to optimize bacterial growth conditions, enhance lysis, and refine purification protocols to boost pDNA yield and purity, ensuring consistent, high-quality results.

Tech Note | An Efficient and Reliable Approach to AAV Packaging
While AAV is a powerful vehicle for gene delivery, unstable ITRs threaten the fidelity of rAAV
genomes. Loss of ITR integrity diminishes the packaging efficiency of rAAV particles and thus
viral titer. In this tech note, discover the effect of partial and full ITR deletions on AAV
packaging and how to reliably identify and effectively correct them.

Tech Note | An Efficient and High-Fidelity Approach to AAV Plasmid Preparation
GENEWIZ has recently developed a proprietary AAV plasmid preparation process, which significantly
improves the chance of maintaining ITR integrity, even when other commercially available
competent cells growing in low temperature have failed. This process can also isolate clones
with full-length ITR from a sample mixture containing intact and truncated ITRs.

Tech Note | AAV-ITR Sequencing
GENEWIZ has developed a high-quality, direct Sanger sequencing method that reads through both
intact and commonly mutated inverted terminal repeat (ITR) regions of adeno-associated virus
(AAV). This method is an effective tool for assessing the integrity of ITRs in AAV plasmids.
Article | Optimizing AAV Plasmid Preparation and ITR Sequencing
The adeno-associated virus (AAV) is a powerful vehicle for gene therapy; however, working with
AAV vectors can be challenging. Spontaneous mutations in the inverted terminal repeat (ITR)
regions can accumulate during plasmid propagation in bacteria. Find out how our scientists have
optimized the AAV plasmid preparation process to deliver 100% accurate constructs.

Workshop & Roundtable Discussion | A Comprehensive Guide to Using AAV Vectors in Gene Therapy
Working with AAV vectors can be challenging. In this on-demand workshop & roundtable
discussion, you’ll gain a better understanding of the AAV development pipeline for gene therapy
research and learn how to optimize upstream and downstream processes including AAV synthesis,
sequencing, bioinformatics, and storage.
