Gene Synthesis & Cloning/Mutagenesis FAQs
The Most Commonly Asked Questions
| Service | Service Offering | Estimated Turnaround Time | Price |
| FragmentGENE | Synthesis of double-stranded DNA fragments | 2-4 business days | $ |
| PriorityGENE | Standard gene synthesis with cloning into a plasmid | 8-10 business days | $$ |
| TurboGENE 5 | Expedited gene synthesis with cloning into a plasmid and our fastest available turnaround | 5 business days | $$$ |
| TurboGENE 7 | Expedited gene synthesis with cloning into a plasmid | 7 business days | $$$ |
| Antibody DNA Synthesis | Synthesis of heavy and light chain sequences with cloning into a plasmid | 6-8 business days | $$ |
| AAV Plasmid Synthesis | Synthesis of AAV sequences and cloning into a plasmid with AAV-ITR sequence verification using proprietary technologies | 11-15 business days | $$$ |
| CRISPR Construct Synthesis | Synthesis of gRNA and cloning into a custom plasmid | 8-10 business days | $ |
| ssDNA Synthesis | Synthesis of single-stranded DNA fragments | 10-15 business days | $$ |
PriorityGENE, TurboGENE, ValueGENE, antibody DNA synthesis, and AAV plasmid synthesis services require you to request a quote before ordering. FragmentGENE, CRISPR construct synthesis, and ssDNA synthesis services are available for direct order, so you do not need to request a quote for these services.
Log in to your online GENEWIZ account and select “Gene Synthesis”, then click on the name of the specific service you are interested in. You will be directed to a quote request form for that selected service. Once you complete the form, select the “Review Inquiry” button at the bottom of the screen. After you review the entered details, select the “Submit Quote Request” button to request your quote. Upon submission of the inquiry, you can expect to receive a quotation within one business day. You can view a PDF copy of the order information including price and download using the “Print Price” button.
The assembled full-length gene is cloned into the pUC-GW-Kan/Amp vector via the EcoRV site. The final construct is verified with both Sanger DNA sequencing (on at least one strand) and restriction digestion. The sequences of pUC-GW-Kan/Amp can be found here.
We currently process in-house pUC-GW-Kan/Amp plasmids at our facility free of charge. As such, we request that a 5 µg aliquot of circularized plasmid (min. concentration of 20 ng/µl) is provided to our facility. This sample can be directly shipped at room temperature to the address below, or it can be submitted via any available GENEWIZ dropbox. To ensure the sample is correctly identified, please include the first page of the order receipt or quotation in your package. If those documents are not available, the project tracking number is acceptable as well.
Azenta
Attn: Project Management
115 Corporate Boulevard
South Plainfield, NJ 07080
USA
We store any starting material provided and final constructs generated at our facility for up to two years to be used for future orders. For more information, please find our Sample Storage Policy here.
The timeline will depend on the size of the gene to be synthesized, the complexity of the vector for cloning, and the requested DNA preparation scale. For reference, the turnaround time for a gene less than 1.5 kb is 8-10 business days with our PriorityGENE service, 7 business days with TurboGENE 7 priority, or 5 business days with TurboGENE 5 priority. A more specific turnaround time for each order is included within the quotation, as well as the “Order Review” page within your online account.
GENEWIZ sample tubes are delivered containing 2-5 µg lyophilized plasmid DNA for gene synthesis orders, or 500 ng – 1 µg purified linear dsDNA for FragmentGENE orders. For resuspension, we recommend the following:
a. Spin the tube briefly to ensure that the contents are on the bottom of the tube
b. Resuspend the sample in a volume appropriate for your needs:
i. For example, 20-50 µL for ~0.25 to ~0.1 µg/µL of sterile high purity water
c. The resuspension solution is dependent on downstream applications – TE may interfere with various enzyme manipulations
d. Vortex briefly, let the DNA tube sit for 2-10 minutes on ice, then vortex again
ORDERING
Alternatively, please feel free to download our Ordering Template and fill out the necessary information within this form. To access this form within a gene synthesis inquiry, select the “Download/Upload” button located at the top right corner of the screen. After this is done, select the same “Download/Upload” button to view the option to upload the completed form.
Select the “Review Quote” button next to the related project tracking number (30-xxxxxxxxx). After this is done, an “Add to Cart” button will appear at the bottom of the screen. Once this is selected, you will be redirected to the ordering page where you will be able to review our Terms and Conditions and enter the payment information for this order. After this is done, select the “Confirm” button at the bottom of the screen to place your order.
Our Project Management Team is happy to help with any questions. Please feel free to contact us from 9:00 AM – 6:30 PM EST via phone (+1-908-222-0711 ext. 3) or contact us here.
Our antibody DNA synthesis service provides synthesis and cloning of your antibody heavy/light chain sequences up to 1.5 kb into any custom vector in as few as 6 business days, the fastest turnaround time on the market. Our PriorityGENE service provides synthesis, assembly, and cloning of sequences with unlimited length on a timely basis.
With our AAV plasmid synthesis service, we can synthesize and clone transgene expression cassettes into custom AAV vectors with high efficiencies. All final products will come bundled with mini-scale or large-scale DNA preparation using our new AAV plasmid preparation protocol and AAV-ITR sequence verified AAV plasmids. This service also offers ITR correction if mutations are found after sequence-verification of these regions.
SERVICE DETAILS
GENEWIZ from Azenta will synthesize cloned gene sequences ranging from 1 bp to 50+ kb, using short DNA oligos as building blocks.
GENEWIZ from Azenta has a proven track record of synthesizing difficult templates, such as GC-rich sequences and sequences with repeats, using our proprietary technologies. With our bioinformatics platform, GENEWIZ optimizes your gene for synthesis.
In some very rare instances, extreme sequence content may be refractory to available sequence verification techniques. GENEWIZ carefully reviews every sequence to estimate difficulty and anticipate project challenges and customizes every project quotation to include an estimated turnaround time and pricing information based on this analysis.
GENEWIZ from Azenta provides a custom quote for every gene synthesis project. For standard priority, typical gene sequences (non-repetitive, average GC-content) that do not require custom subcloning, no additional fees will apply. For genes with expedited handling, difficult sequences, large inserts, or for projects with custom subcloning requirements, any relevant fees will be indicated in your custom gene synthesis quote.
At GENEWIZ from Azenta, the protection of our customers’ intellectual property is a top priority. Proprietary information is always under client ownership, and Azenta places the utmost importance on confidentiality and privacy. Comprehensive protective measures enable Azenta to earn and maintain the trust and confidence of all customers, collaborators, and partners. For more information about the Azenta Confidentiality Policy, please click here.
As the variants are being created from the parent sequence, we can offer a discounted price for variant synthesis. Please keep in mind, variant synthesis does add an additional 3-5 business days to the quoted turnaround time.
RECOMBINANT ANTIBODY PRODUCTION
Yes, we offer a pETE vector which is optimized for our recombinant expression platform. You can provide your own preferred expression vector as well.
Expression analysis is performed by BLI (Octet) to estimate the amount of protein in the supernatant. Non-producing plasmids will be reported to the customer and excluded from purification, so you do not pay purification fees on non-producing constructs.
Standard deliverables include: 2-5ug synthesized DNA construct (if performed by GENEWIZ), antibodies provided as purified protein or supernatant, Certificate of Analysis (CoA)
AAV
GENEWIZ requires either the sequence of your gene of interest (GOI) or a full-length transfer plasmid to provide AAV particles.
If providing a GOI sequence, we’ll synthesize and clone the gene into your custom vector. For submitting a custom vector, please provide at least 5 ug of starting material normalized to >200 ng/ μL.
If you prefer to directly submit the plasmid, please submit 1 mg per 1E13 vg target yield.
AAV packaging capacity is typically ~4.7 kb (inclusive of the ITRs from ITR-ITR). We can package larger cargo, but this is typically associated with a reduction in titer and transduction efficiency.
In vivo grade GENEWIZ ultra-purified AAV particles are produced by simultaneous transfection (triple transfection) of suspension HEK293T cells with RepCap, Helper, and transfer plasmids; the transfer plasmid contains your gene of interest. Virus is then purified by iodixanol density gradient ultracentrifugation, followed by buffer exchange and concentration to the final volume.
In vitro grade AAV particles are produced by the same triple transfection process, but they are purified using only PEG precipitation and centrifugation, and do not undergo iodixanol purification.
Viral particles are delivered on dry ice, suspended in buffer, with a certificate of analysis. Our default delivery format is up to 20 aliquots of viral particles. For any number of aliquots >20, there is an added charge of $5 per aliquot.
AAVs are delivered on dry ice and should be stored immediately at –80°C. When stored under these conditions, AAVs can last several years. Multiple freeze-thaw cycles are not recommended.
Viral titer and endotoxin measurement are both part of standard quality control for AAV packaging. The viral titer is determined by qPCR with ITR primers, and endotoxin measurement of <10 EU/mL is determined by an LAL test. As an add-on QC, <1 EU/mL is available.
Yes, we offer dPCR or ddPCR as optional add-on quality control services.
Yes, we offer TEM, which can measure the empty and full capsids, as an optional add-on quality control service. Please note that this does not distinguish between full and partial capsids; if partial capsid determination is necessary, we recommend NGS viral genome analysis.
The production yield varies based on the production scale and viral serotype. As an example, most 1 mL orders for a high-yield serotype are 1E13 vg.
The standard titer is 1E13 GC/mL for ultra-purified virus.
Yes, we do routinely offer packaging of AAV libraries, specifically for ITR containing transfer plasmids. Many customers will also characterize the diversity of the virus using our viral sequencing with NGS.
If you are interested in capsid library packaging, we can take those projects on a case-by-case basis depending on the diversity of the library. Please inquire with our project management team during your consultation.
Yes, this is offered for volumes of 250 μL and 500 μL at 1E11-1E12 GC/mL.
LENTIVIRUS
Lentivirus, meaning “slow virus”, is a genus of retrovirus. Retroviruses express reverse transcriptase to convert their RNA genome into double-stranded DNA (dsDNA) and integrase to incorporate this viral DNA into the host’s genome. Due to this permanent DNA integration, the host cell produces more retroviruses as it divides, resulting in long-term expression of the disease.
Lentivirus has long incubation periods between host infection and the onset of disease symptoms, such as human immunodeficiency virus (HIV) in the case of human acquired immune deficiency syndrome (AIDS). In biotechnological applications, recombinant lentiviral vectors are modified for safe handling and used as a gene delivery vector for mammalian cells.
Unlike other retroviruses that only infect dividing cells, lentiviruses infect both dividing and non-dividing (postmitotic) cells. This broad tropism, coupled with the stable integration into a host cell genome, allows for diverse in vitro and in vivo applications, including clinical cell therapy and CAR-T cell engineering. Additional advantages of lentivirus include the ability to express large transgenes (up to 9 kb) and low immunogenicity.
Our team of experts uses HEK293T cells for lentiviral vector production. Following successful transfection with four packaging plasmids, the cell machinery facilitates the assembly and replication of the lentiviral particles during an approximately three-day incubation. Lentivirus particles are then harvested, filtered, purified, concentrated, and aliquoted to small volumes to facilitate your downstream transduction applications.
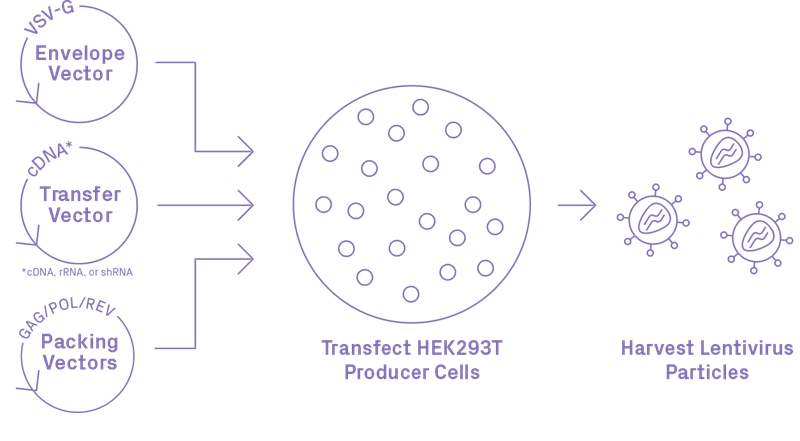
Recombinant lentiviral vectors are generally considered safe as they are engineered to be replication incompetent, with the third-generation considered the safest. It features a chimeric 5’ LTR, self-inactivating truncated 3’ LTR, elimination of Tat, and a four-plasmid transfection system that has a low likelihood of generating a replication-competent virus. According to the guidelines of the National Institutes of Health (NIH), lentiviral particles should be handled as Risk Group 2 (RG2). Our team uses the third-generation packaging system in biosafety level 2 (BSL2) facilities for all default lentiviral handling.
Yes! Upon review, our expert team can accommodate custom requests. Our default option is an envelope glycoprotein of vesicular stomatitis virus (VSV-G protein).
Yes! Our team of experts have extensive experience packaging various library types, including combinatorial and trimer-controlled libraries. The diversity of the final packaged lentivirus will depend on the plasmid material. If you do not already have a library sample, we can generate and validate this for you. Please see here for more information.
Yes! We offer various transfer plasmid options for your cloning needs in the context of lentivirus production. If we don’t have a vector that suits your requirements, we can synthesize any necessary components to create a transfer plasmid that aligns with your design.
We offer two QC options for the validation of your lentiviral particles:
1. p24 ELISA (physical titer): This method, offered as our standard QC, utilizes a sandwich ELISA to detect the presence of p24 protein, a major capsid protein encoded by the Gag gene. The p24 protein level is measured spectrophotometrically against a standard curve. Next, the quantified p24 value is correlated to the virus particle number. To facilitate downstream applications, we convert the lentiviral particles (LP) to TU/mL by dividing by 100 (there are ~100-1000 LPs for every active virus). This method is considered as a measure of physical titer.
2. WPRE qPCR (functional titer): An aliquot of the packaged lentivirus vector is used to transduce HEK293T cells at various dilution levels. After a two-day incubation period, the genomic DNA of the transduced cells is harvested. Following that, qPCR amplification of the WPRE element is then performed alongside a calibrated standard curve. The WPRE element is absent in wild-type HEK293T cells but exists within the lentiviral cassette of a transfer plasmid. As the WPRE elements can only be detected in HEK293T cells if transduction is successful, this method is considered as a measure of functional titer.
Physical titering approximates the total amount of virus present in the sample by correlating it to the spectrophotometrically quantified p24 in an ELISA assay, regardless of whether it possesses the gene of interest or ability to infect cells.
Functional titer is a measure of virus infectivity, quantifying the copy number of the WPRE element, which is detectable only upon a successful viral DNA integration into the host cell genome. Generally, functional titer is 100-1000-fold lower than physical titer. We report physical titer after accounting for this ratio, and express p24 in TU/mL.
Pseudotyping is the process of incorporating envelope glycoproteins from other viruses to generate lentivirus particles with altered tropism. This is often used to enhance the infectivity and stability of lentivirus. For example, VSV-G is commonly used for pseudotyping due to its broad tropism.
CODON OPTIMIZATION
GENEWIZ codon optimization tool can optimize for multiple critical parameters to stabilize DNA fragments and improve gene expression efficiency. Parameters include, but are not limited to:
a. Codon usage bias
b. GC content
c. mRNA secondary structure
d. Custom desired patterns
e. Custom undesired patterns
f. Repeat sequences (direct repeat, inverted repeat, and dyad repeat)
g. Restriction enzyme recognition sites (deletion or insertion)
Please note, GENEWIZ from Azenta cannot guarantee final protein expression.
Yes, we offer dual optimization for any two expression systems requested. Additional requirements are also considered for review by the project team. Please note that any dual-system optimization represents a compromise, and a sequence specifically optimized for any given system may outperform the compromise sequence. To add this to your order, please add the request to the “Order Comments” section of your gene synthesis inquiry or email the Project Management Team at gs@azenta.com.
CLONING AND MUTAGENESIS
We currently only provide our in-house pUC-GW-Kan/Amp vectors for cloning. If you’d prefer cloning into a different vector, an aliquot of your custom vector will need to be provided upon confirmation of your order.
For any information regarding on-going promotional discounts, please contact your sales representative.
| Site-Directed Mutagenesis | Insert PCR-Based Mutagenesis | TurboMUTANT | |
| Plasmid Size Guidelines | 10kb or less in size, Non-complex |
> 10 kb/unstable plasmid | < 8 kb/stable plasmid |
| Turnaround Time | Starts from 8-10 BD | Starts from 10-15 BD | Starts from 5 BD |
QUALITY AND FINAL DELIVERABLES
Standard deliverables for GENEWIZ gene synthesis projects include:
a. 2-5 µg of lyophilized plasmid containing your desired synthetic construct
b. Certificate of Analysis (COA), including restriction digest
c. Sequence trace data with alignment
d. Sequence of synthetic gene alone and in the designated vector
All reagents, material used, and equipment are kept under strict quality controls by the lab. The Project Management Team may decide to implement additional controls if an uncommon failure rate is observed.
Mutations can be found at multiple stages of a project. For example, a synthesized primer may contain an incorrect base. Mutation correction may delay delivery by 1-4 business days depending on the situation.
The GENEWIZ Project Management Team is happy to address your additional project requests through a new quotation request that references the current open project. Where possible, any discounts applied to the current project will be incorporated into the new quotation. Please note that you must confirm the new project within the ongoing project timeline in order to take advantage of these discounts.
Yes, unless otherwise specified in the quotation due to extreme complexity. Single-strand sequence verification across the entire insert occurs for every synthesis product. If the sequence-verified synthesis product is associated with a large-scale preparation, the product from the large-scale plasmid preparation is sequenced across junctions. Additional sequence verification on the second strand, or after large-scale plasmid preparation, is available if requested (additional charges apply).
Some extremely complex sequences are expected to not be verifiable using current Sanger sequencing techniques and are noted in the quote. In the very rare event where the Project Management Team is not able to deliver the intended material, there will be no charges for your project (unless otherwise specified in the quotation due to extreme complexity).