CLIA Sanger Sequencing
GENEWIZ Multiomics & Synthesis Solutions from Azenta Life Sciences provides CLIA Sanger sequencing as the clinical “gold standard” from our state-of-the-art CLIA-certified and CAP-accredited laboratory while following Good Clinical Practices (GCP) guidelines. This standalone sequencing service utilizes a CLIA-compliant workflow to support your clinical diagnostic assays, leading to advancements in personalized medicine and molecular diagnostics.
CLIA Sanger sequencing is best utilized as the initial readout from a PCR assay or in conjunction with next generation sequencing, specifically to elucidate complex regions not approachable by other methodologies or to confirm variants uncovered by genome-wide techniques.
Features And Benefits
CLIA Sanger Sequencing Service Options
Pre-Mixed
For sequencing plasmids or purified PCR products, provide your template and primer mixed in the same tube for the most economical CLIA-compliant services.
Pre-Defined
If choosing our Pre-Defined Service, submit your plasmids or purified PCR products and primer in separate tubes, and a Azenta scientist will mix them once your sample arrives on site.
Custom
Full-service option allows you to submit unpurified PCR product with unknown concentration along with your primer for sequencing. We will purify your PCR product as well as measure and adjust the concentration to optimize your reactions.
Please view our Sample Submissions Guidelines
–>Template Types

Purified Plasmids or PCR Products

Un-Purified PCR Products

Difficult Template Sequencing
Deliverables
All customers will receive their raw data as .ab1, .seq, and .phd files along with a quality score report.
HOW TO ORDER
Email | Phone (1-877-436-3949, Ext. 3350)
HOW TO ORDER
Email | Phone (1-877-436-3949, Ext. 3350)
Direct Colony Sequencing
GENEWIZ’s Direct Colony Sequencing services utilizes rolling circle amplification (RCA) to enable the Sanger sequencing of bacterial clone or phage sample templates without the need for plasmid preparation. RCA generates DNA for sequencing by random hexamer priming of circular templates. GENEWIZ RCA protocols can handle sequencing projects of any size, and are optimized to produce high quality reads from both bacterial colonies and glycerol stocks as well as phage plaques and supernatants. Direct Colony Sequencing is also extremely effective when low amounts of DNA starting material are available (e.g. low copy plasmids).Place Order Place an Order

RCA Sequencing
-
 1
1 -
-
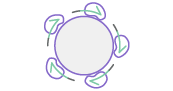 2
2 -

-
 3
3 -

-
 4
4
Dean, Frank B. et al. “Rapid Amplification of Plasmid and Phage DNA Using Phi29 DNA Polymerase and Multiply-Primed Rolling Circle Amplification.” Genome Research 11.6 (2001): 1095–1099.
Direct Colony Sequencing Features & Benefits
RCA Sequencing Applications
shRNA cloning and library screening; Eliminate plasmid preps and sequence colonies directly.
DNA methylation analysis: Save time & obtain high-quality reads by cloning PCR products directly and sequencing the bacterial colonies.
Antibody phage display library validation: Direct phage sequencing for faster screening and validation.
SAMPLE SUBMISSION OPTIONS
Sample Submission Options
| Glycerol Stock* | Send 1.5 ml tubes or 96-well plates; samples must be shipped on dry ice. Please label with media type. |
|---|---|
| Bacterial Colonies* | Array colonies in a grid on the agar plate and circle the colonies requiring sequencing. Numerically label each colony. Note that unnumbered plates will be processed as random pick. If using liquid culture, submit samples in 96-well plate with strip caps. Wrap agar plates in parafilm, and provide a cushion to protect against potential damage. |
| Phage Supernatant* | Please submit 50 ul of phage supernatant in 8-strip PCR tubes or 1.5 ml microcentrifuge tubes clearly labeled with sample name. |
| Phage Plaque | Circle and number the plaques you submit for sequencing. For transport, wrap agar plates in parafilm and provide cushion to protect against potential damage. |
*Please note: RCA protocol is intended for circular templates <10 kb; linear templates will not be amplified sufficiently for direct Sanger sequencing.
Longer circular templates, or circular templates carried in bacterial strains with functional endA may not be reliably amplified, contributing to variable results
Please visit our Sample Submission Guidelines for bacteria and phage templates for additional details.







